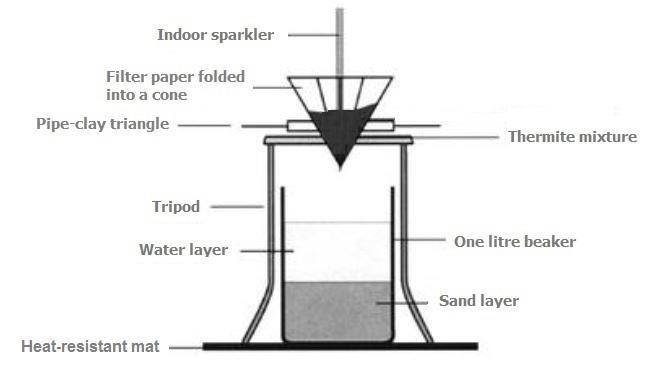
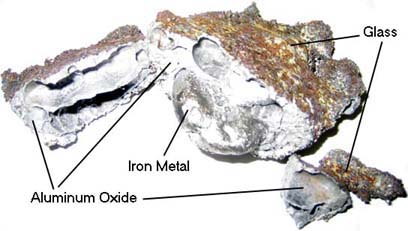
Thermit reaction, iron (III) oxide reacts with aluminium andgives molten iron and aluminium oxide. - YouTube

Corundum Is A Crystalline Form Of Aluminium Oxide Typically Containing Traces Of Iron, Titanium, Vanadium. It Is A Rock-forming Mineral. 3d Illustration Stock Photo, Picture And Royalty Free Image. Image 60744188.
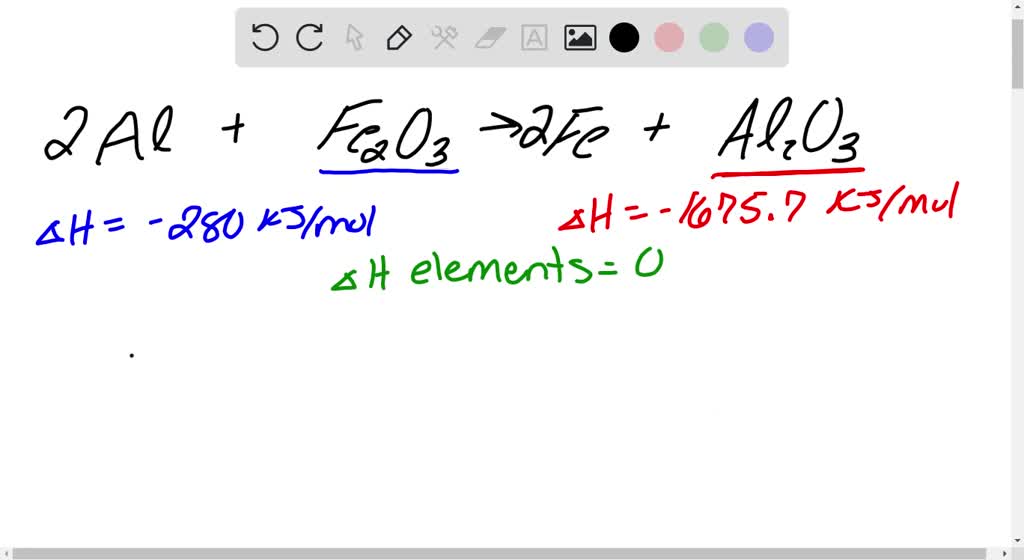
SOLVED: When aluminium metal reacts with iron (III) oxide a single displacement reaction occurs to form iron and aluminum oxide. Using the Standard Enthalpy Chart write the balanced chemical equation and calculate
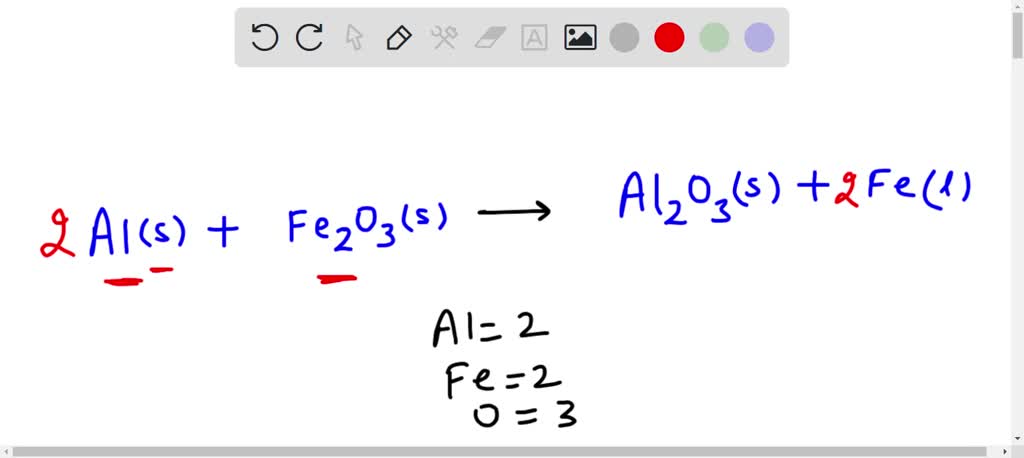
SOLVED: Write a balanced chemical equation based on the following description: the reaction of powdered aluminum and powdered iron(III) oxide produces solid aluminum oxide and liquid iron metal

Iron iii oxide react with aluminium and give molten iron and aluminium oxide write a balanced chemical - Brainly.in

SOLVED: Question 14 4 pts Given the following standard enthalpy changes of formation iron(Il) oxide; FeO(s) = -266 kJ/mol aluminium oxide; AlzO3(s) = -1676 kJ/mol aluminum; Al (s) = 0 kJ/mol iron,