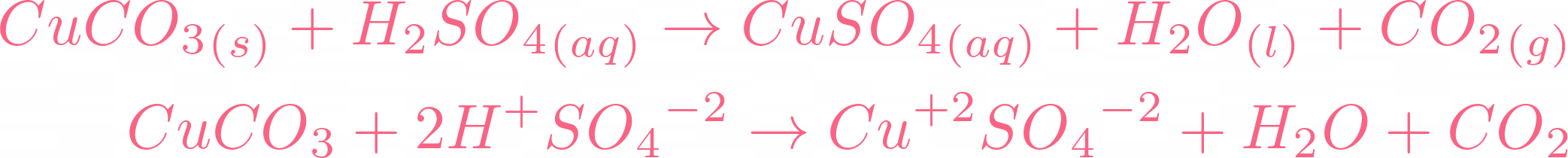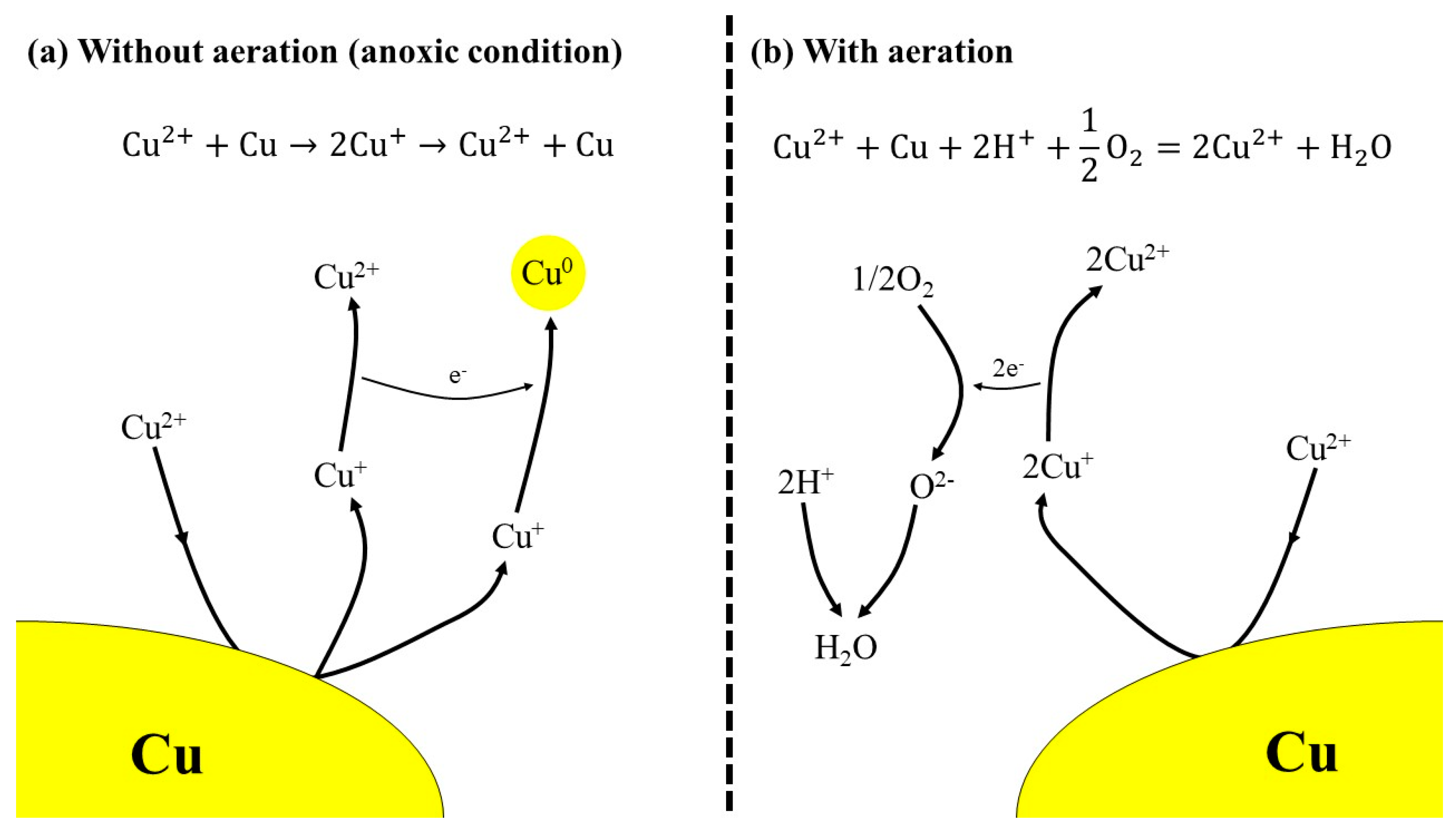
Metals | Free Full-Text | Improvement of Copper Metal Leaching in Sulfuric Acid Solution by Simultaneous Use of Oxygen and Cupric Ions

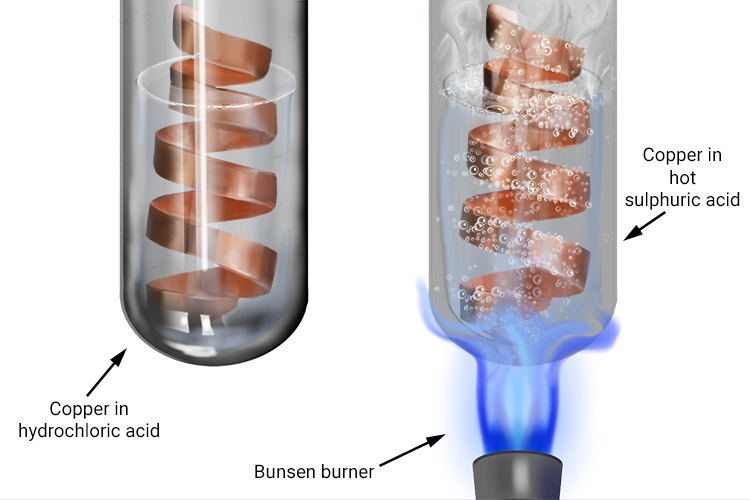
Predict reaction of 1n sulphuric acid with copper lead and iron - Chemistry - Electrochemistry - 13647999 | Meritnation.com
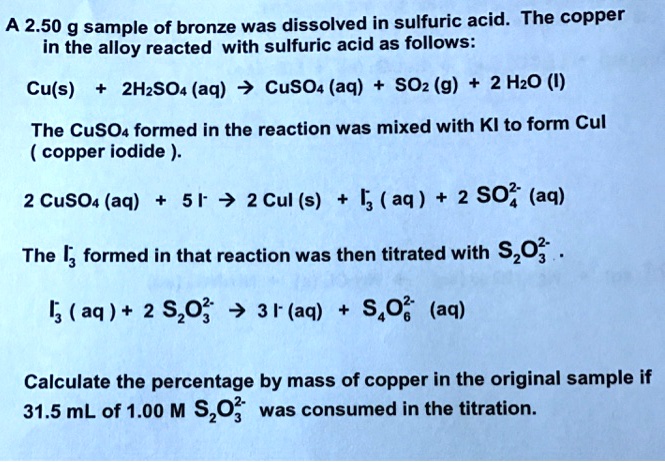
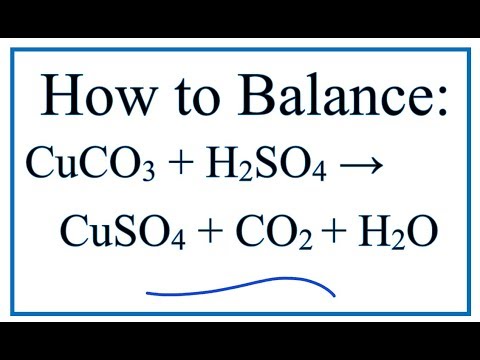
SOLVED: A 2.50 g sample of bronze was dissolved in sulfuric acid: The copper in the alloy reacted with sulfuric acid as follows: Cu(s) 2HzSO4 (aq) CuSOa (aq) SOz (g) 2 HzO ()

See the reaction between concentrated sulphuric acid and copper. Cu^@+2H2^(+1)S^(+6)O4^(-2) → Cu^(+2)S^(+6)O4^(-2)+S^(+4)O2^(-2)+2H2^(+1)O^(-2) Which is the oxidizing agent in this reaction? Which is the reducing agent?
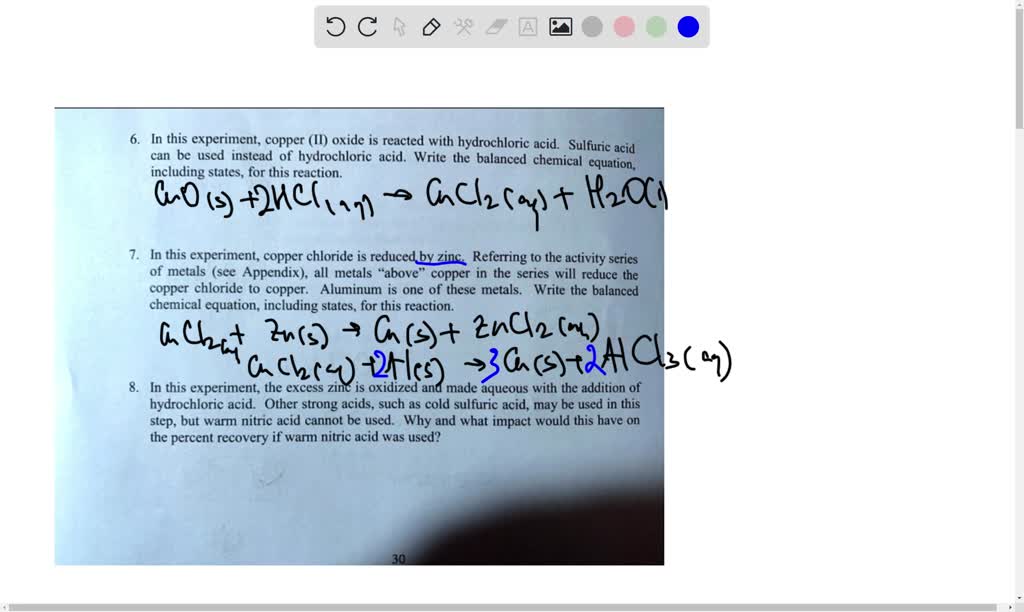
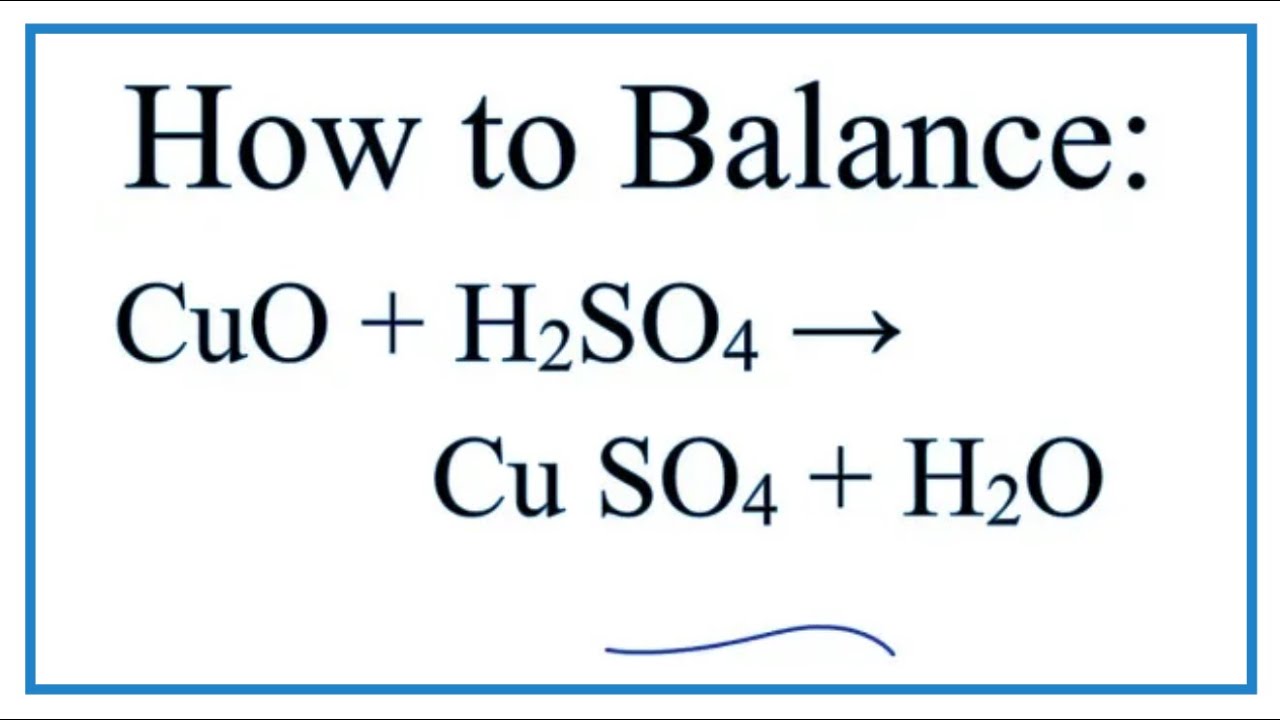
SOLVED: In this experiment, copper (II) oxide is reacted with hydrochloric acid. Sulfuric can be used acid instead of' hydrochloric acid Write the balanced chemical equation; including states, for this reaction In
![The percentage of water of crystallisation in hydrated Copper Sulphate [CuSO4.5H2O] is:(Cu = 63.5, S = 32, O = 16, H = 1) The percentage of water of crystallisation in hydrated Copper Sulphate [CuSO4.5H2O] is:(Cu = 63.5, S = 32, O = 16, H = 1)](https://dwes9vv9u0550.cloudfront.net/images/5171946/16be827c-5f80-410b-a9f7-0243530e09a9.jpg)
The percentage of water of crystallisation in hydrated Copper Sulphate [CuSO4.5H2O] is:(Cu = 63.5, S = 32, O = 16, H = 1)

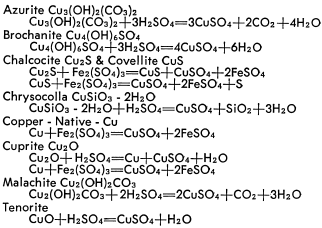


![PDF] Copper Dissoliution in Concentrated Sulfuric Acid | Semantic Scholar PDF] Copper Dissoliution in Concentrated Sulfuric Acid | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/8c23bfe6f390e4c070ab06eca2828378bf1baac3/5-Table1-1.png)